Neuroscientist Jo Anne Stratton, who joined The Neuro last year, is approaching her multiple sclerosis (MS) research from a little-explored angle. She wants to understand how the ependymal cell, which belongs to the glial family of cells, supports brain health and how they might become damaged in people with MS.
“Ependymal cells are the least-studied glial cell,” she says. “Until recently, techniques didn’t exist for distinguishing them from nearby cells. They form a protective barrier between brain matter and the fluid-filled cavities of the brain, called the ventricles. If the barrier breaks down, the balance of fluid and immune cells in the brain gets disrupted and many different issues can arise.”
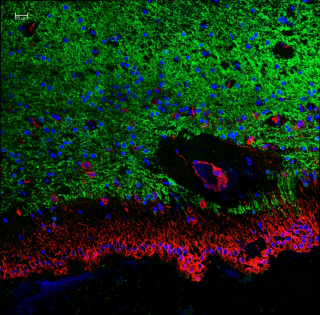
Magnetic resonance scans suggest that ependymal cells become a problem in MS patients. “Some studies have shown that these cells look damaged, and in other studies, they have been shown to completely disappear. But the consequence of this is not known.”
Stratton has created a model in which ependymal cells can be labeled to see how they react to genetic mutations. She is also working on a unique way to grow ependymal cells in a dish from induced pluripotent stem cells (iPSCs), which can be reprogrammed to become any cell in the human body. The use of iPSCs to create artificial cells is critical to neuroscience research because many cells, including ependymal cells, cannot be studied well when still inside the living human brain.
“If we can create an iPSC ependymal model, then we could administer trial drugs to see whether we can reverse any destruction,” she says, adding that the early results are encouraging. “We’re starting with MS, but ependymal damage occurs in other diseases as well.”







