Marta Cerruti talks glowingly of the colourized photos her students recently made. Most researchers would reserve what’s under their microscope for purely scientific inquiry. But while the Associate Professor of Materials Engineering works on ways to grow bones and reduce mineral build-up in arteries, she likes to also look at what’s going on aesthetically. A water colour painter, she recently encouraged her students to make colloidal dispersions, cells and extracellular matrix grown on scaffolds into pieces to admire. “They’re really, really pretty structures.”
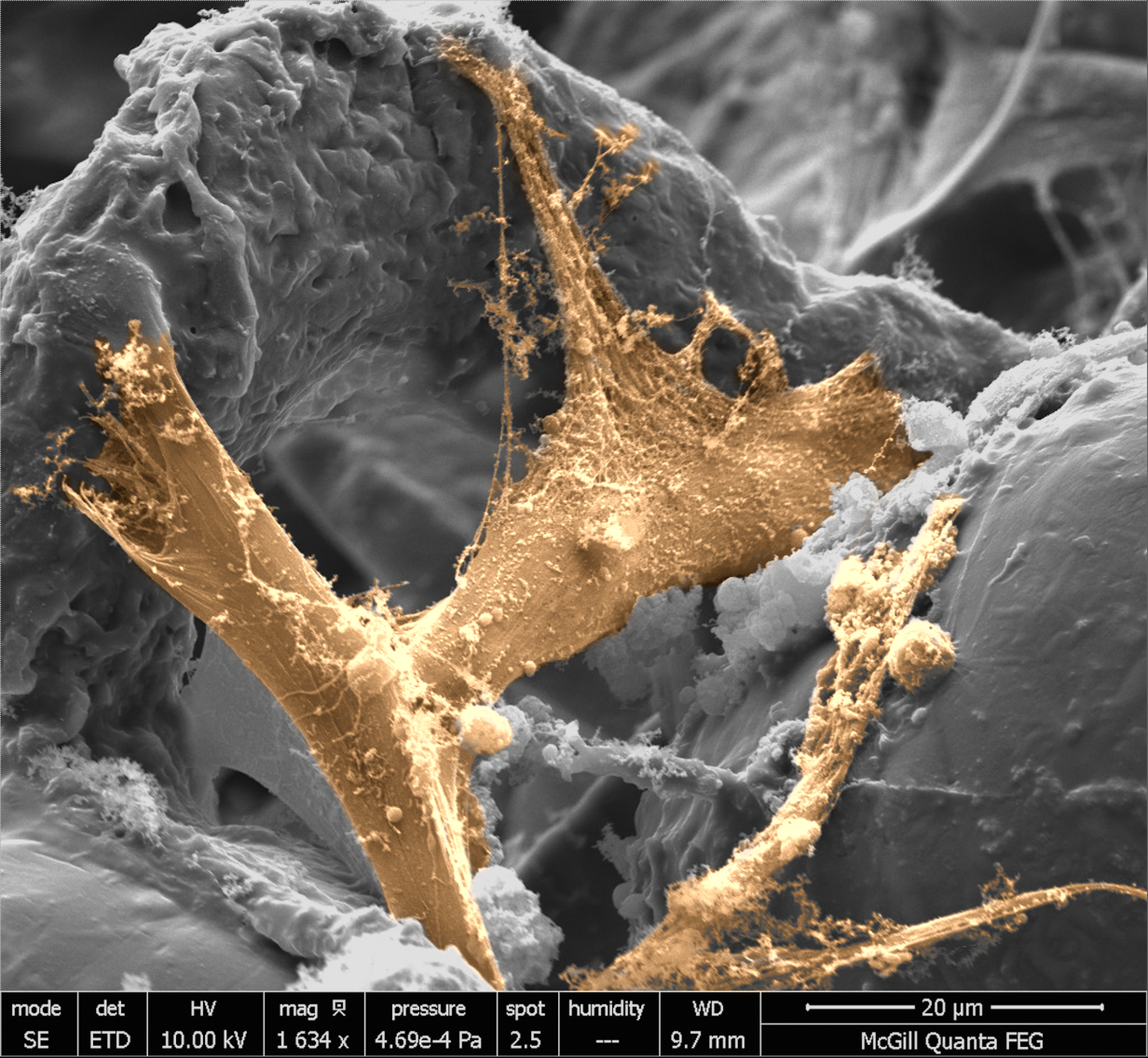
Cerruti’s canvas is wide. She designs scaffolds that coax bone generation but, interestingly, in studying the characteristics of these minerals, she also discovers how to prevent their accumulation in our arteries. Learning how to build is teaching her how to demolish.
Alongside bone generation and mineral analysis, she has also put her mind to the subject of tumours, where the nanoparticles she’s developed hold promise for carrying chemotherapy directly to a cancerous target. The nanoparticles are housed in a hydrogel shell that can carry drugs, while its core is made of a special material that can convert near-infrared light into more powerful UV light and penetrate through skin as deep as two centimetres.
The work on nanoparticles has received a lot of attention after being published in the Journal of the American Chemical Society four years ago, garnering accolades and numerous citations.
“A lot of what we're doing is to prevent side effects of current clinical treatment,” says Cerruti, of the procedure that would avoid releasing chemotherapy into the rest of the body.
Fiorenzo Vetrone, an Assistant Professor of Nanobiotechnology, is an expert in light-emitting minerals and co-authored the paper with her. Like the old TV commercial for Reese’s Peanut Butter Cups, they both discovered how well their two specialties combined: “She's an expert in hydrogels. So, we thought, ‘Why not try and envelope these nanoparticles with a hydrogel?’ which had really never been done,” says Vetrone, who have had students Ghulam Jalani and Vivienne Tam shuttling between them.
“The hydrogels were modified with a molecule that essentially breaks up when it absorbs light,” explains Vetrone. “The light does its thing, and it releases the drug.”
Cerrruti is now working with Bassam Abdulkarim, who is an Associate Professor in the Department of Oncology, a radiation oncologist at the MUHC and an expert in the brain cancer, glioblastoma. “It’s one of the deadliest tumours that exist,” says Cerruti, who sees great promise in using the core-shell nanoparticles to target this hard-to-access tumour in the brain.
Keeping hard minerals away from soft tissues
While we think of materials scientists working with space-age matter, Cerruti’s fundamental studies share more with mineralogists, as she tries to understand calcification and how it builds up in our bodies. She has become the go-to person for identifying the makeup of those minerals found inside arteries. Her work has also helped increase our understanding of how the vessels that transport blood become prone to calcification and how to potentially stop their accumulation.
Blocked arteries usually bring to mind fatty cholesterol-filled deposits, but Cerruti focuses on the rock-like calcification found in them. Calcification, which diminishes the arteries’ role in assisting blood circulation is a fairly widespread condition. It often grows on arteries where fat has already deposited, as well as develops as a result of diabetes, kidney disease, mineral imbalances and certain genetic conditions.
Cerruti says the aging process is one of the likely reasons the arteries, which are primarily made up of elastin, attract calcification. “It seems that degradation of elastin favours calcification.” But there are also situations where calcification takes place without any degraded elastin.
“So how can we block these sites that are attracting calcium?” asks Cerruti.
One answer may lie in her ability to classify minerals. She has identified minerals that are at their early stages of forming into calcification. That may make them easier to remove with novel therapies.
If medication is to be used, another question from Cerruti is “How do you get the drug to destroy the calcification in the artery without destroying the bone?”
She and collaborator Monzur Murshed, Associate Professor in the Faculty of Medicine’s Division of Endocrinology and Metabolism, have been looking at a peptide that can act as a mineralization inhibitor, one more way her practice is using targeting. Murshed explains that he and Cerruti foresee a potential procedure where the peptide would be placed in a capsule and introduced in the bloodstream. “The capsule will have a specific docking molecule and will go and find that partner molecule in the target tissue and will dock there and slowly release its contents to inhibit pathologic calcification.” Students Abhinav Parashar, Ophelie Gourgas, Tao Song and Kirk Lau have also been part of the collaboration.
Home-grown bones
These minerals are not just blocking arteries; they are the building blocks of bones. So, it makes sense that a researcher who tries to keep calcification out of soft tissue, will also look for ways to harness those minerals to see how they could rebuild and grow bone.
Cerruti designs scaffolds that bone cells can glom on to and begin the process of growing into bone. Individuals who have lost large pieces of bone though cancer or trauma have traditionally needed to have bone grafted from one part of their body onto the damaged area, or use artificial implants like titanium. But having your own body’s healing mechanisms to fill in those missing pieces avoids the painful procedures that currently exist.
Cerruti and her colleagues have, in fact, been getting bone cells to transform into new bone. So, with those kinds of advancements being made by her lab and so many others, what, Cerruti is asked, have been some of the commercial applications coming out of the research?
“You want to know the truth?” she deadpans. “Zero.”
It appears that the 30-year-old field of tissue engineering, especially bone tissue engineering, has been struggling to make viable products. The industry has been floundering for a host of reasons, including the cost of the procedures, the difficulty of replicating the complex architecture and weight-bearing properties of bone, the unforeseen responses of the immune system and health regulator roadblocks.
Cerruti’s eyes are wide open to the scientific challenges she faces. “It’s very hard to make material that will be able to call in cells from your own body and get them to start colonizing the scaffold.”
But she and her colleagues soldier on.
She has made strides, says collaborator Lisbet Haglund, Associate Professor of Surgery. “The material that she's developed, it works. The cells adhere, they make a calcified matrix,” says Haglund, who is well aware that the real test will be when the project moves from lab to animal. “The body’s ability to take the cells through their different phases cannot be replicated in the lab. The goal is to have a material that, when you put it in the body, can simulate the natural process.”
Cerruti, once again has involved a team, which includes students Dhanalakshmi Jeyachandran (See her video of the work: https://bit.ly/2YEftLS) and Prof. Haglund, and, for different kinds of scaffolds, Yiwen Chen and Electrical Engineering professor Thomas Szkopek. They are preparing to place Cerruti’s bone-attracting scaffolds and their bone cells into animals, beginning with it being inserted under the skin to study compatibility and blood-flow issues. Perhaps, she may move that stuck commercial needle a few measures ahead.
Math was bred in the bone
Marta Cerruti was born in northern Italy 41 years ago and grew up one of two daughters of university math professors. When she was around six years-old, her father would quiz her and her sister on math word problems.
“At some point, I got very good with the problems that were about filling bathtubs with water. You know, you have a faucet that’s filling the bathtub at this speed and a hole that is draining water at that speed, and how long before it overflows?” she recalls, adding that her sister was particularly skilled at train problems, where speeds and times of departure would help to figure out when the two would collide head-on.
Her parents left lots of books around for their children, with Marta, as a young child, picking up titles on insects and animals, and then later poetry, such as Dante’s The Divine Comedy. There was much discussion in the house on culture and science.
More recently, her mother’s math credentials led to her tutoring Christopher Havens, an inmate who in 2011 received a 25-year sentence for murder in Washington State. Her father got involved, as well, and Haven’s math skills improved to the point of him having been recently published in the journal Research in Number Theory. Marta published a piece in the Conversation [https://bit.ly/2ZgfhkT] about their experience. She was heartened by some of the math community’s reactions to the piece and put Havens in touch with several different professors who wanted to help him.
It also got her thinking more about the lack of opportunities faced by incarcerated individuals who want to pursue math and science. She would like to help start a local chapter of Walls to Bridges, a Canadian organization that provides university courses for those serving time in penitentiaries.
Cerruti’s work with colleagues is akin to a group expedition of seemingly different experts, all bringing along their collective know-how. Whether it’s a molecular biologist, a molecular geneticist or a nano-bioscientist, she seeks out their talent and pursues collective goals.
“It's this interface between what we can do and what we are,” she says about the interdisciplinary work.
That eclecticism and open-mindedness has also led her to plan a project with Concordia Fine Arts professor Alice Jarry. The two recently received a Petro-Canada Young Innovator Award that will provide seed funding for a piece that looks at membranes for air filtration and brings together both the scientist and artist perspectives.
Her science career began with that same out-of-the-box thinking, when she worked on NovaMin Technology for her PhD. It’s the trade name for the bioglass used to help tooth enamel remineralize and is an ingredient in some formulations of Sensodyne toothpaste. She explains the process of delivering extra ions to the enamel and helping the saliva in its efforts to precipitate mineralization. “We were able to show that in a few minutes you could create this calcium phosphate. There was enough data to convince them that it would be a good idea for toothpaste.”
After her Ph.D., she spent two years at North Carolina State University and another two at UC Berkeley. She joined McGill in 2009 and, since 2011, has been Canada Research Chair in Biosynthetic Interfaces. She and her common-law husband are raising a girl, 7, and a boy, 4.
Most recently, she adapted her work to meet head-on the Covid-19 crisis. She helped develop an application for fabric that will neutralize the pandemic’s virulent droplets. The project is a collaboration with the chemical company Blachford and recently received NSERC funding.
“Because there's a huge shortage of personal protective equipment, the idea is to have something that can be very easily applied to a non-N95 mask or even a scarf and have a protection that would completely prevent viral infection because the virus would be killed upon contact with this coating.”
She calls it virus proofing.
The McGill researcher seems to thrive from having so many projects on the go but there is a cohesive sense to it all. So, an art piece is born from a scaffold that coaxes bone to grow. That structure is also key to breaking down minerals that harden arteries. Those minerals find their way to arteries, just like hydrogels she designs and hopes will find their way to tumours.
This all has potential to truly help society, the same way that her antiviral coating can help a community struggling with a pandemic. Meanwhile her math matchmaking helped an inmate also struggling, who found some freedom through publishing his math problems. Math problems? That’s where it all started, with a girl inspired by equations around how fast a bathtub could overflow. And those were part of the bigger equations that got worked out and would eventually add up to a fascinating career.
