Optogenetic stimulation of the netrin-1 DCC receptor in HEK293T cells
By: Hanna Davies
Supervisor: Dr. Tim Kennedy

Correct development of the central nervous system is essential for normal cognitive function. The precise wiring of the brain is a crucial element of this development. Netrin-1 and two of its receptors, Deleted in Colorectal Cancer (DCC) and un-coordinated (UNC-5), are proteins that play a crucial role in guiding axons to their final targets (10). The function of netrin-1 and its receptors are necessary for normal development, as the absence of netrin-1 results in embryonic lethality (1). Netrin-1 continues to play an important role after development, in the adult brain. Specifically, netrin-1 activated DCC signaling has been shown to be involved in synaptic plasticity, as well as in spatial and recognition memory (3, 12). Accordingly, aberrant netrin-1 and DCC signaling results in a decrease in brain connectivity and has been linked to neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (7, 11).
Netrins are chemotropic guidance cues (4). A gradient of netrin-1 will act to either attract or repel axons depending on the type of netrin-1 receptors present on the cell surface (8). The interaction between netrin-1 and DCC promotes an attractant effect, such that axons will grow towards higher netrin-1 concentrations. In contrast, the interaction between netrin-1 and an UNC5/DCC complex repels axons away from high netrin-1 concentrations (8). These gradients are important for development and have been the focus of much of the research on the netrin-1 protein. However, there is increasing evidence that these proteins are also vital for the proper functioning of the adult brain (3, 10, 12). Studies investigating the role of these proteins in the adult brain have focused on techniques that lack precise spatial or temporal regulation such as cell cultures, brain slices and mice knockout models. A significant path of future inquiry is to study the role of netrin-1 and its receptors in behaviour, through precise spatiotemporal manipulation of these proteins. With the discovery of optogenetics, a technique that uses light to manipulate protein function, there is now an opportunity to pursue these advanced behavioural studies (5)(6).
The chemoattractant effects of netrin-1 on axonal outgrowth are mediated by the homodimerization of two neighbouring DCC subunits (8). Three years ago a modified version of DCC, titled DCC photo-activatable (DCC-PA), was synthesized to dimerize in response to light at a specific wavelength (440-488 nm) (2). This photo-activatable protein allows for optogenetic control of DCC activation and could be used to induce synaptic plasticity as well as modulate memory in an in vivo model with spatiotemporally regulated light stimulation. However, before moving forward with this new technique the effects of light activated DCC-PA must be compared to the effects of endogenous netrin-1 activated DCC.
One of the most prominent effects of netrin-1 activated DCC is the structural change that have been observed in DCC expressing cell cultures after bath application of netrin-1. Namely, netrin-1 causes an exponential increase in the number of cell outgrowths (9). These structural changes have been observed in both neuronal cultures and in Human Embryonic Kidney (HEK293T) cells modified to express the DCC protein (9). In our study, we investigated the downstream effects of DCC-PA by comparing the structural outgrowth resulting from netrin-1 activated DCC with that resulting from light activated DCC-PA.
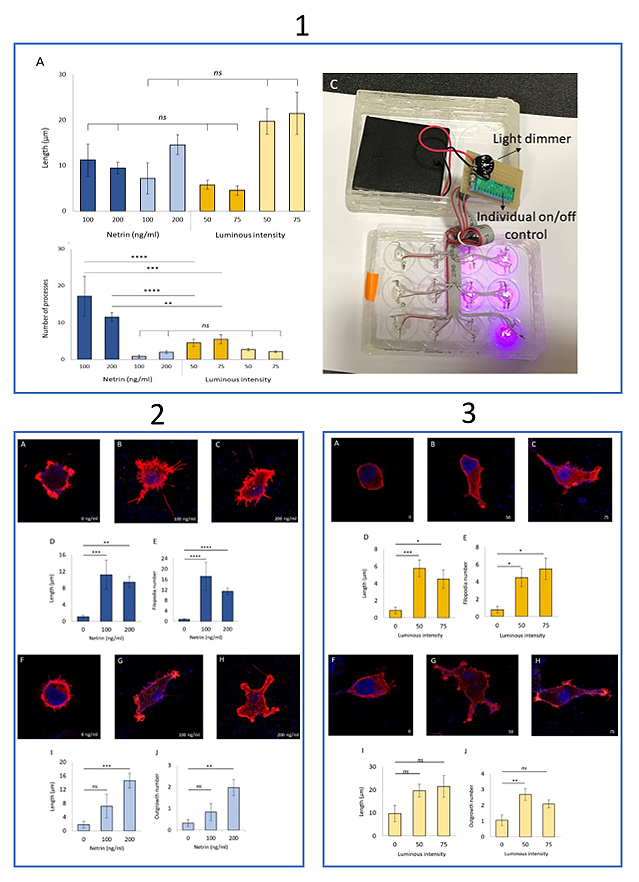
Structural changes were compared in HEK293T cells transfected to express either endogenous DCC or modified DCC-PA. A twelve-well, blacked out, glass bottom plate was used to culture the differentially transfected cells in separate wells of the same plate. We custom built a photo-stimulator apparatus using LED lights that emit the optimal light wavelength and a circuit board was created to control which wells were photo-stimulated during incubation. In addition, we added a dial to allow for control of light intensity (Fig. 1c). Two types of structural changes were classified in our experiments, thicker outgrowths and thinner filopodia. The number and length of the two types of processes were compared with their respective controls; controls were cells expressing the same protein but that had not been treated with netrin-1 or light.
In our study, both netrin-1 activated DCC and light activated DCC-PA conditions demonstrated a significant increase in number and length of filopodia relative to controls (Fig. 2d, 3e, 3d, 3e). Similarly, there was a significant increase in the number of outgrowths for both conditions but only the netrin-1 activated DCC condition showed a significant increase in outgrowth length (Fig. 2i, 2j, 3i, 3j). These results show that both conditions lead to similar structural changes. We then compared the strength of the effect between the two conditions. Specifically, we investigated if one condition led to more structural changes than the other. We found that netrin-1 activated DCC led to significantly more filopodia outgrowth. However, all other structural growth was comparable in number and length (Fig. 1a, 1b). We also experimented with increasing the light intensity using the built in intensity dial and decreasing the netrin-1 concentration. In contrast to what was expected, we found that experiments done with the dial set to 75% full light intensity actually resulted in less emitted intensity than those done with the dial set to 50% as measured by a photometer. As experiments with the dial set to 75% were done last, we believe the decrease in light intensity was due to overuse of the LED lights.
Taken together, our findings suggest that in HEK293T cells light activated DCC-PA leads to similar, but not identical, downstream structural changes as endogenous netrin-1 activated DCC. Differences between the two conditions may be due to off target effects of either netrin-1 or the light. Netrin-1 has many receptors which may have their own downstream cascades that could affect changes in cell structure. Alternatively, optogenetics is a newly emerging technique and may have unknown off target effects (10). These findings offer valuable data which can be used in future studies investigating the role of netrin-1 in behaviour, neural plasticity, different forms of memory and the potential involvement of netrin-1 and DCC in debilitating neurodegenerative diseases.
Click image for larger version.
Figure 1. (A) There was no significant difference in the filopodia (darker shades) and outgrowth (lighter shades) lengths of DCC expressing HEK293T cells in the presence of netrin-1 (blue) and PA-DCC expressing HEK293T cells in the presence of light (yellow). This relationship remained regardless of light intensity and netrin-1 concentration. (B) DCC expressing HEK293T cells in the presence of netrin-1 had significantly more filopodia (dark blue) than the HEK293T cells with PA-DCC exposed to light (dark yellow) the significance of this difference varied depending on the concentration of netrin-1 and the light intensity present. There was no significant difference in the number of outgrowth in the two conditions (light colors) regardless of light intensity or netrin-1 concentration. Error bars are SE. Statistical significance was determined by a one-way ANOVA followed by Tukey’s post hoc test for multiple comparison, ns P> 0.05, * P< 0.05, ** P<0.01, *** P<0.001. 100 ng/ml netrin-1 (A, B) n = 6, 200ng/ml netrin-1 (A, B) n= 32, 50% intensity photo-stimulation (A, B) n= 26, 75% intensity photo-stimulation (A, B) n= 12. (C) The photo-stimulation device used to activate PA-DCC.
Figure 2. Netrin-1 increases the number and length of filopodia and outgrowths in DCC expressing HEK293T cells. HEK293T cells were transfected with constructs encoding full-length DCC (A-C and F-H). Expression of transfected full-length DCC construct was visualized using purified mouse anti-human DCC and anti-mouse IgG cross-absorbed secondary antibody alexa Fluor 555 (red). HEK298 nucleus and F-actin structures were visualized using Hoechst (blue) and Alexa Fluor 633 phalloidin (not shown), respectively. DCC expressing HEK 293T cells were cultured either in absence (A, F) or presence of purified recombinant chick netrin-1 at concentrations of 100ng/ml (B, G) or 200ng/ml (C, H). Filopodia length and number was quantified and compared in (D) and (E) respectively. The same comparison was done for outgrowth length (I) and number (J). White arrows in (B and C) indicate model filopodia measured and arrows in (G and H) instead indicate examples of outgrowths measured and counted. Images were taken with 63x objective lens. Error bars are SE. Statistical significance was determined by a one-way ANOVA followed by Tukey’s post hoc multiple comparisons test where: * P < 0.05, ** P < 0.01, *** P<0.001, **** P<0.0001. Without netrin-1 (A, F) n= 19, 100 ng/ml netrin-1 (B, G) n= 6, 200ng/ml netrin (C, H) n= 32.
Figure 3. HEK293T cells expressing PA-DCC photo-gated receptor in the presence of light (440nm) showed an increase in number and length of filopodia present as well as an increase in outgrowth number. HEK293T cells were transfected with constructs encoding PA-DCC construct (A, B, C, F, G, H). Expression of transfected PA-DCC protein was visualized using purified mouse anti-human DCC and anti-mouse IgG (H+L) cross-absorbed secondary antibody Alexa Fluor 555 (red). HEK298 cell nucleus and F-Actin structures were visualized using Hoechst 33342 and Alexa Fluor 633 phalloidin respectively. PA-DCC expressing HEK 293T cells were cultured in absence (A, F) or presence of 440nm light at 50% intensity (B, G) or 75% intensity (C, H). As done in the DCC netrin-1 model, filopodia length and number was quantified and compared in (D) and (E) respectively and outgrowth length and number we also quantified and compared in (I and J). White arrows in (B and C) indicate model filopodia measured and arrows in (G and H) instead indicate examples of outgrowths measured and counted. Images taken with 63X objective lens. Error bars are SE. Statistical significance was determined by a one-way ANOVA followed by Tukey’s post hoc test for multiple comparison where: * P < 0.05, ** P < 0.01, *** P<0.001, **** P<0.0001. Without photo-stimulation (A, F) n = 13, 50% intensity photo-stimulation (B, G) n = 26, 75% intensity photo-stimulation (C, H) n = 12.
References
- Bin, J. M., Han, D., Sun, K. L. W., Croteau, L. P., Dumontier, E., Cloutier, J. F., ... & Kennedy, T. E. (2015). Complete loss of netrin-1 results in embryonic lethality and severe axon guidance defects without increased neural cell death. Cell reports, 12(7), 1099-1106.
- Endo, M., Hattori, M., Toriyabe, H., Ohno, H., Kamiguchi, H., Iino, Y., & Ozawa, T. (2016). Optogenetic activation of axon guidance receptors controls direction of neurite outgrowth. Scientific reports, 6, 23976.
- Horn, K. E., Glasgow, S. D., Gobert, D., Bull, S. J., Luk, T., Girgis, J., ... & Hamel, E. (2013). DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell reports, 3(1), 173-185.
- Kennedy, T. E., Serafini, T., de la Torre, J., & Tessier-Lavigne, M. (1994). Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell, 78(3), 425-435.
- Kramer, R. H., Mourot, A., & Adesnik, H. (2013). Optogenetic pharmacology for control of native neuronal signaling proteins. Nature neuroscience, 16(7), 816.
- Lima, S. Q., & Miesenböck, G. (2005). Remote control of behavior through genetically targeted photostimulation of neurons. Cell, 121(1), 141-152.
- Lin, L., Lesnick, T. G., Maraganore, D. M., & Isacson, O. (2009). Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends in neurosciences, 32(3), 142-149.
- Moore, S. W., Tessier-Lavigne, M., & Kennedy, T. E. (2007). Netrins and their receptors. In Axon Growth and Guidance (pp. 17-31). Springer, New York, NY.
- Shekarabi, M., & Kennedy, T. E. (2002). The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Molecular and Cellular Neuroscience, 19(1), 1-17.
- Sun, K. L. W., Correia, J. P., & Kennedy, T. E. (2011). Netrins: versatile extracellular cues with diverse functions. Development, 138(11), 2153-2169.
- Van Battum, E. Y., Brignani, S., & Pasterkamp, R. J. (2015). Axon guidance proteins in neurological disorders. The Lancet Neurology, 14(5), 532-546.
- Wong, E. W., Glasgow, S. D., Trigiani, L. J., Chitsaz, D., Rymar, V., Sadikot, A., ... & Kennedy, T. E. (2019). Spatial memory formation requires netrin-1 expression by neurons in the adult mammalian brain. Learning & Memory, 26(3), 77-83.