Research Projects
The mechanism of the force generation by myosin II motor studied by High-Speed Atomic Force Microscopy
Myosin molecular motors are fundamental to various cell functions that are essential for cell survival, including cell division, organelle trafficking, and cell motility. All these tasks require energy to be performed, which the molecular motors generate by converting the chemical energy of ATP hydrolysis into mechanical work and force generation. Therefore, understanding the force-generating steps produced by myosin II is essential. However, there are still several mechanistic details that are not fully understood due to the limitations of current technical capabilities in visualizing rapid changes in non-processive molecular motors.
To overcome these limitations, we utilize high-speed atomic force microscopy (HS-AFM) to visualize the actin-myosin complex at high temporal and spatial resolutions, which enables us to gain further insights into the myosin mechanism of force generation. By applying HS-AFM, we can directly observe myosin II's working steps, and we can investigate the structural changes that occur during the mechanochemical cycle. The ability to observe the actin-myosin complex at such high resolutions allows us to analyze the molecular mechanics involved in force generation, enabling us to construct more detailed and accurate models of the mechanism of myosin's action.
In summary, the application of high-speed atomic force microscopy offers a powerful tool for understanding the mechanisms of myosin II's force generation. By directly observing the working steps of myosin, we can gain further insight into the processes underlying the generation of force and build more accurate models of the molecular mechanics involved in cellular processes such as cell division, organelle trafficking, and cell motility.

The relationship of force production, actin motility and the force-velocity relation produced by myosin II filaments and myofibrils.
The force of muscle contraction is created by the interaction between myosin and actin. Our research focused on how the load and the relationship between force and velocity are affected by myosin filaments interacting with actin, as well as the role of surface loops in the myosin molecule in regulating this interaction. We developed a method to study the effects of load on the ability of isolated muscle myosin filaments to generate force, which can be used to investigate various muscle-related issues and diseases. Additionally, we used a limited form of proteolysis to remove different amounts of the loops 1 and 2, the ATP-loop, and the actin-binding domain from heavy meromyosin (HMM). We found that the movement of actin propelled by myosin and the percentage of actin filaments moving decreased when loop 1 was depleted from HMM. Additionally, the ATPase activity also decreased with the depletion of loops 1 and 2. Our findings suggest that loop 1, in coordination with other surface loops, plays a key role in regulating the velocity of actin movement in response to load. This highlights the importance of the flexible loops in the structure of myosin in regulating the interaction between myosin and actin.

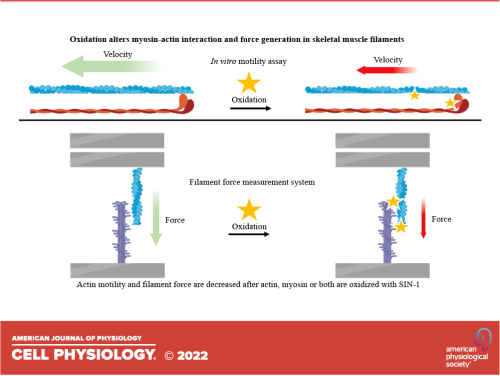
The effects of oxidation on contractile properties of skeletal muscles.
The goal of this study is to investigate the effect of actin and myosin oxidation on the interaction between actin and myosin, which is the basis of contraction and force production in muscle fibers. Previous studies have shown that actin and myosin oxidation cause myofibrillar weakness in healthy and diseased muscles, but the degree to which oxidation of each of these proteins contributes to an attenuated force in myofibrils is unclear.
The study will use two methods to measure the effects of oxidation on actin-myosin interactions: an in vitro motility assay (IVMA) and a cantilever-based filament force measurement system (FFMS). The IVMA will be used to measure the F-actin sliding velocity over heavy meromyosin (HMM) before and after treatment with the chemical 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride (SIN-1), an NO and O2•− donor. The FFMS will be used to measure the force production by thick and thin filaments before and after treatment with SIN-1.
The study will observe that the motile velocity of actin propelled by myosin decreases when either actin or HMM is oxidized with SIN-1. Additionally, the study will observe that the filament forces are reduced when either thick or thin filaments are oxidized. The study will conclude that oxidation of both myosin and actin affects contractile events at the molecular level, which may ultimately lead to a decrease in force generation in skeletal muscles.