1-15
Fig. 1
Diagrammatic representation of the testis showing a seminiferous tubule and the rete testis, the ductuli efferentes, the epididymis and vas deferens. The shaded regions indicate areas of the different segments of the epididymis, i.e., the initial segment, caput, corpus, and proximal and distal cauda, where data on the relative quantitative distribution of the major different epithelial cell types were obtained.
Fig. 2
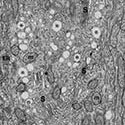
Light microscope photograph showing the sharp demarcation between the cuboidal epithelial cells of the rete testis (RT) and the columna epithelial cells of the efferent ducts (ED)
Fig. 3
Portion of the epithelium from the initial zone of an efferent duct at the light microscopic level showing numerous nonciliated cells (NC) and few ciliated cells (C)
Fig. 4
Portion of the epithelium from the terminal zone of an efferent duct showing nonciliated (NC) and ciliated (C) cells
Fig. 5
Light microscope photograph showing several tubules of the initial segment of the epididymis
Fig. 6
Portion of the epithelium lining the initial segment of the epididymis illustrating many tall columnar principal cells (P) with pale, round nuclei (n) located at different levels of the cell and showing a prominent nucleolus, a few elongated basal cells (B) at the base of the epithelium, and a deeply stained narrow cell (N). Longitudinal dense bands extend along the length of principal cells (arrowheads)
Fig. 7
Epithelium lining the epididymal duct of the caput epididymidis showing numerous principal cells (P) and a few clear cells (C)
Fig. 8
Principal (P) and clear (C) epithelial cells lining the caput epididymidis
Fig. 9
Epithelium lining the epididymal duct of the corpus epididymidis showing numerous principal (P) cells and a clear (C) cell
Fig. 10
Principal (P) and clear (C) epithelial cells lining the corpus epididymidis.
Fig. 11
Epithelium lining the epididymal duct of the cauda epididymidis showing principal (P) and clear (C) cells
Fig. 12
Principal (P) and clear (C) epithelial cells lining the cauda epididymidis
Fig. 13
Relative cell distribution in the initial segment, caput, corpus, and cauda epididymidis of the adult male rat
Fig. 14
Portion of the epithelium (E) lining the proximal segment of the vas deferens showing many cuboidal principal cells and a clear cell (C)
Fig. 15
Epithelium of the proximal segment of the vas deferens lined by cuboidal principal (P) cells and flattened basal (B) cells
16-30
Fig. 16
Portion of the epithelium (E) lining the distal segment of the vas deferens
Fig. 17
Tall columnar principal (P) epithelial cells lining the distal segment of the vas deferens showing a brush border (asterisk) and moderately stained nucleus (n) usually in the midregion of the cell
Fig. 18
A diagrammatic representation of a nonciliated cell from the terminal zone of the ductuli efferentes
Fig. 19
Apical and supranuclear regions of a nonciliated cell from the initial zone of the efferent ducts
Fig. 20
Apical and supranuclear regions of a nonciliated cell from the terminal zone of the efferent ducts
Fig. 21
High-power electron micrograph of the apical region of a nonciliated cell. Tubular coated pits (cp) connected to the apical plasma membrane can be seen extending into the cell cytoplasm from the bases of the microvilli (Mv)
Fig. 22
High-power electron micrograph of the apical region of a nonciliated cell. Tubular coated pits (cp) presumed to be still connected to the cell surface are evident
Fig. 23
High power of the apical region showing numerous apical tubules (T) with a uniform, moderately dense staining content, some of which can be seen to be connected (arrowheads) to large dilated spherical membranous bodies (asterisks), showing an empty, pale stained lumen lined by a fuzzy material
Fig. 24
A large vacuole, containing a fine flocculent material, referred to as an endosome (E) is seen in the apical region to which is connected an apical tubule (T, arrowhead)
Fig. 25
Junction between the apical and supranuclear regions of a nonciliated cell of the terminal zone
Fig. 26
Supranuclear region of a nonciliated cell showing several homogeneous dense lysosomes (L)
Fig. 27
A membrane-bound body deep in the supranuclear region of a nonciliated cell showing features of a dense lysosome (L) and lipid (LIP) delimited by a common unit membrane
Fig. 28
A membrane-bound body next to the nucleus (N) of a nonciliated cell showing features of a dense lysosome (L) and lipid (LIP)
Fig. 29
A well-defined tubular network of a nonciliated cell formed of short anastomosing tubes demarcating pores of various sizes (asterisks) is seen to be connected at several sites (small arrowheads) with the lateral plasma membrane (PM)
Fig. 30
Low-power electron micrograph showing tall columnar ciliated cells (C) and adjacent nonciliated cells (NC)
31-45
Fig. 31
High-power electron micrograph of the apical region of a ciliated cell presenting basal bodies (B), cilia (c), a few small coated and uncoated pits (arrowheads), apical tubules (T), numerous subsurface vesicular profiles, pale multivesicular bodies (MVB), glycogen granules (circled), and filaments (f)
Fig. 32
High power of the supranuclear region of a ciliated cell containing several membrane-bound dense bodies identified as lysosomes (L)
Fig. 33
Diagrammatic representation of a principal epithelial cell from the initial segment of the epididymis
Fig. 34
Apical region of a principal cell from the initial segment of the epididymis showing large coated and uncoated pits of the cell surface (large arrowheads), numerous cisternae of the sparsely granulated endoplasmic reticulum (ER), several large smooth-surfaced vesicles showing a patchy flocculent material (asterisks) and the occasional small coated and uncoated vesicles (small arrowheads)
Fig. 35
High-power electron micrograph of the apical region of a principal cell from the initial segment of the epididymis. Numerous cisternae of the sparsely granulated endoplasmic reticulum (ER) containing a unifom filamentous material are evident, some of which can be seen in close proximity to the apical plasma membrane (arrows)
Fig. 36
Supranuclear region of a principal cell of the initial segment of the epididymis
Figs. 37-38
Supranuclear region of a principal cell of the initial segment showing the Golgi apparatus formed of several stacks of saccules (S) seen in close association with the sparsely granulated endoplasmic reticulum (stars)
High power of a Golgi stack of saccules (S)
Fig. 39
Basal region of a principal cell of the initial segment
Fig. 40
Diagrammatic representation of a principal cell of the caput epididymis
Fig. 41
High power of the apical region of a principal cell of the caput epididymis
Fig. 42
High power of the apical region of a principal cell of the corpus epididymis
Fig. 43
Apical region of a principal cell of the cauda epididymis
Fig. 44
Supranuclear region of a principal cell of the cauda epididymis showing an elaborate Golgi apparatus
Fig. 45
Supranuclear region of a principal cell of the caput epididymis
46-61
Fig. 46
Basal region of a principal cell of the caput epididymis
Fig. 47
Apical and supranuclear regions of a principal cell of the corpus epididymis
Fig. 48
Supranuclear region of a principal cell of the corpus epididymis
Fig. 49
Basal region of a principal cell of the corpus epididymis demonstrating an abundance of lipid droplets (LIP)
Fig. 50
Principal cell of the cauda epididymis
Fig. 51
A principal cell of the cauda epididymis showing a slender foot-like process contacting the basement membrane (arrow) and interdigitations (arrowhead) with a basal cell (B)
Fig. 52
A principal cell of the distal segment of the vas deferens
Fig. 53
High power of the supranuclear region of a principal cell seen in Fig. 52
Fig. 54
High power of the basal region of a principal cell seen in Fig. 52
Fig. 55
Apical region of a principal cell of the vas deferens showing coated pits (Cp), large coated vesicles (Cv), smooth-surfaced vesicles (asterisks), and small coated (arrows), and uncoated (arrowhead) vesicles
Fig. 56
The supranuclear region of a principal cell of the vas deferens
Fig. 57
High power of a Golgi stack of saccules (S) showing on its cis face (C) interruptions of the saccules forming wells (arrows) in which are located small vesicles
Fig. 58
A montage of a pencil cell within the epithelial lining of the vas deferens
Figs. 59-61
Apical and supranuclear regions of a narrow cell of the initial segment of the epididymis
High power of the apical region of a narrow cell showing numerous small C-shaped vesicles often appearing as double-walled structures (arrowheads) and glycogen granules (circles)
High power of the apical region of a narrow cell containing a pale multivesicular body (asterisk) and numerous small C-shaped vesicles (arrowheads)
62-75
Fig. 62
Montage of a clear cell of the cauda epididymis
Figs. 63-65
High power of the apical region of a clear cell of the cauda epididymis showing a large coated pit (Cp) and large coated vesicle (CV), numerous small uncoated vesicles (v), and a large vacuole (V)
High power of the apical region of a clear cell of the cauda epididymis
Supranuclear region of a clear cell of the cauda epididymis
Fig. 66
A basal cell of the caput epididymis, showing an elongated nucleus (N) enclosed by a small amount of cytoplasm containing a Golgi apparatus (G)
Fig. 67
A basal cell of the cauda epididymis
Fig. 68
A halo cell in the lamina propria of the epididymis enclosed by the arms of a myoid cell (MY)
Fig. 69
A halo cell in the basal region of the epididymal epithelium
Fig. 70
Appearance of different organic solutes in the lumen of the rat and hamster (*) proximal caput, distal caput (a) epididymis (▲), or cauda (■) epididymidis after systemic infusion of the radioactive compound, followed by direct micropuncture or microperfusion of the duct
Fig. 71
Schematic representation of the probable steps in endocytosis, transcytosis, and secretion in principal cells of the epididymis and vas deferens
Fig. 72
Structure of testosterone, dihydrotestosterone, and 5α-androstan-3α,17β-ol and their enzymatic interconversion in the rat epididymis
Fig. 73
Glutathione S-transferase activity in isoelectric focused peaks of sections of the epididymis-vas deferens
Fig. 74
Concentration (mM) of sodium, potassium, chloride, and phosphate in the luminal fluid of rat seminiferous tubules (SNT), rete testis, head (caput), body (corpus) and tail (cauda) of the epididymis
Fig. 75
Densitometric pattern of luminal proteins from the rete testis and epididymis of rams separated by one dimensional SDS polyacrylamide gel electrophoresis
76-90
Fig. 76
Number of condensed sperm heads in the rat testis (striped), caput ( ), and cauda (dots) epididymidis as a function of age
Figs. 77-78
Cross-section through the cytoplasmic droplet (CD) of a sperm located in the lumen of the caput epididymidis
The cytoplasmic droplet (CD) of a sperm located in the lumen of the corpus epididymidis. In this case the droplet appears to be in the process of being shed from the flagellum (F)
Fig. 79
Lumen (Lu) of the cauda epididymidis
Fig. 80
A spermatozoon (S) present in the lumen (Lu) of the cauda epididymidis
Fig. 81
A residual body (RB) located in the lumen (Lu) of the corpus epididymidis, identified by the presence of many mitochondria (m), large vacuoles (V ), and clusters of ribosomes (arrowhead)
Fig. 82
Schematic representation showing the position of the cytoplasmic droplet of spermatozoa in the testis and various segments of the epididymis and its ultimate fate
Fig. 83
Transit time in days for spermatozoa to traverse the caput (shaded), corpus (clear) and cauda (striped) epididymidis
Fig. 84
Approximate site within the epididymis where spermatozoa acquire their fertilizing ability
Fig. 85
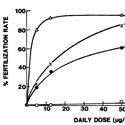
Effect of increasing doses of testosterone (●), dihydrotestosterone (X), 5α-androstan-3α, 17β-diol (Δ), and 5α-androstan-3β, 17β-diol (o), injected daily, on the maintenance of the fertilizing ability of spermatozoa in the ligated hamster cauda epididymidis 1 week after castration
Fig. 86
Subcellular localization of 5α-reductase activity during development in the caput-corpus and cauda epididymidis
Fig. 87
Effects of various lipids (A) and phosphatidylcholines with defined acyl structure (B) on the specific activity of rat epididymal 5α-reductase
Fig. 88
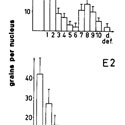
Grain number over principal cell nuclei of mouse epididymis after injection of tritiated dihydrotestosterone (DHT) or tritiated estradiol (E2)
Fig. 89
Fertilizing ability of spermatozoa from the distal cauda epididymidis of the rabbit as a function of time after either castration or hypophysectomy (HYPOX)
Fig. 90
Formation of 5α-reduced metabolites of testosterone by isolated rat caput epididymal principal cells incubated for 15 hr in serum-free medium at various temperatures ranging from 25 to 40ºC