Ozonolysis reactions: impact on atmospheric oxidation
Mechanisms of ozonolysis reactions are very complex and still poorly understood. Our theoretical studies suggested that during the dark seasons, ozonolysis of alkenes are the major sources of HO (the dominant "atmospheric detergent"), H2O2 and RO2 in the atmosphere. This reaction pathway is indeed calculated to be much more important than the "traditional" photochemically initiated reactions during dark seasons. We have also shown that this additional amount of HOx can be major cause of conversion of Sulfur (IV) to Sulfur (VI), a phenomenon which is also so-called "acid-rain" in atmospheric water. Our laboratory studies provided temperature dependence kinetic data set and peroxide yields for over 15 internal and terminal alkene systems and provided structure-reactivity relationships, implemented in atmospheric chemical models. We aimed to obtain a better understanding of the chemical mechanisms for the formation of condensable material from gas phase organic compounds, as well as the chemical composition, reactivity and volatility of the particles formed. Our group addresses these questions from the precursor end. We study kinetics and mechanisms of the oxidation reactions by using spectroscopic and chromatographic techniques to follow the changes in concentration of the precursor compounds and major intermediates. We have indeed developed a moving solid phase microextraction unit to the long path FTIR, used periodically at different reaction steps for better analysis of organic particles, along with a multi-stage aerosol impactor. The experiments are supported by theoretical studies of possible intermediate species. The results are used to develop parameterizations of oxidation mechanisms for use in computer models. Another area of our ongoing research includes understanding the ozononlysis reactions of alkenes and its subsequent reactions with water and water dimer. The ozone-initiated oxidation of alkenes is among the few reactions of closed shell molecules (non-radicals) leading to the formation of free radical species in the atmosphere. Criegee intermediate (CI) was postulated as intermediate in the ozonolysis reactions of alkenes in the liquid and gas phases (Eq. 1).
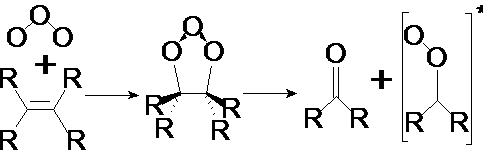
We have determined the temperature dependence kinetics for some key ozonolysis reactions and developed structure-reactivity relationship for predictive use. We have also performed theoretical studies including the reactions of parent and substituted Criegee intermediates with water and the water dimer. According to our calculations, the most favourable route is the formation of hydroxyalkyl hydrogen peroxide as the result of the reactions of CI with the water dimer. Our ongoing and near future research entails theoretical investigations of the reactions of CI with water clusters, and laboratory mechanistic studies of alkenes and ozone at different environmental conditions, including relative humidity and temperature. We also perform reactions reactions at surfaces (e.g., Hung and Ariya, J. Phys. Chem., 2007). There is no overlap with the projects in the enclosed proposal.
Selected related publications in this domain
- E. D. Hudson, and P. A. Ariya, Measurements of Non-Methane Hydrocarbons, DOC in Surface Ocean Waters, and aerosols over the Nordic Seas during Polarstern cruise ARK-XX/1, Chemosphere, DOI:10.1016/j./chemosphere./2007.04.056 (2007)
- H. M. Hung, P. A. Ariya, The Oxidation of Oleic Acid and Oleic Acid/Sodium Chloride(aq) Mixture Droplets with Ozone: the Changes of Hygroscopicity and the Role of Secondary Reactions, /J. Phys. Chem. A.,/ 111(4); 620-632 (2007)
- Ryzhkov, Andrew B. and Ariya, Parisa A., A theoretical study of the reactions of carbonyl oxide with water in atmosphere: the role of water dimer. Chemical Physics Letters, 367(3-4), 423-429 (2003).
- E. Avzyanova, P. A. Ariya, Kinetic studies of ozonolysis of selected terminal and internal alkenes: evaluation of HO yield, International Journal of Chemical Kinetics, Volume 34, Issue 12, 678-684 (2002).
- P. A. Ariya, R. Sander and P. J. Crutzen, Significance of HOx formation in the winter-time: A modelling studies, Journal of Geophysical Research, 105, 17721-17738 (2000).