We’ve moved
The Rosalind and Morris Goodman Cancer Institute can now be found at www.goodmancancer.ca
The Rosalind and Morris Goodman Cancer Institute can now be found at www.goodmancancer.ca
Professor/Professeur
Rosalind and Morris Goodman Cancer Institute
Distinguished James McGill Professor
Departments Oncology and Biochemistry
Faculty of Medicine and Health Sciences

Dr. Tremblay has been recognized at each stage of his scientific career by prizes and salary support awards that recognize his excellence as a cancer researcher.
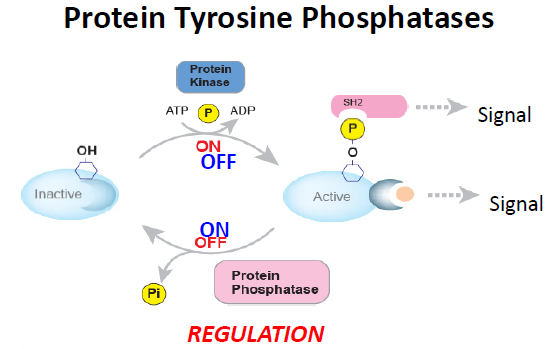
Dr. Tremblay’s laboratory investigates the functions of several members of the protein tyrosine phosphatase family (PTP) in the following ways:
1) Characterization of five of the PTP family by gene knock-out technologies and other molecular approaches - The PTPs are a large family including over a hundred gene members. As they serve as controls for each other functionally and experimentally, Dr. Tremblay chose to work simultaneously on several of these enzymes. Our research revolves around the following five PTPs; PTP-PEST (PTPN13), PTP1B (PTP-N1), TC-PTP (PTPN2), the LAR-PTPs subfamily and especially PTP-sigma (RPTPS), and the oncogenic PRL2 (PTP4A2).
These enzymes remove the phosphate moiety from tyrosine and were considered tumor suppressor genes counteracting the protein tyrosine kinase family, of which over 70% have been associated with oncogenic activities. Yet, our laboratory and others have clearly shown that many PTPs are as oncogenic as tumor suppressors. Particularly important for us is the oncogenic role of PTP1B and PRL2 in breast and other cancers. Using gene knock-out technologies and other molecular approaches we generated various mouse models and assays for all these enzymes and demonstrated their functions in cancer and also in other human diseases.
PTP1B is a prime target in type II diabetes, obesity, and dyslipidemia, TC-PTP is involved in hematopoietic stem cells expansion, DNA damage response and IBD/ colitis, PTP-PEST interacts with several proteins important in inflammatory and arthritis syndromes and PTP-sigma plays a role in axonal extension during development, axonal regeneration after a spinal cord injury and in ureterocele in animals.
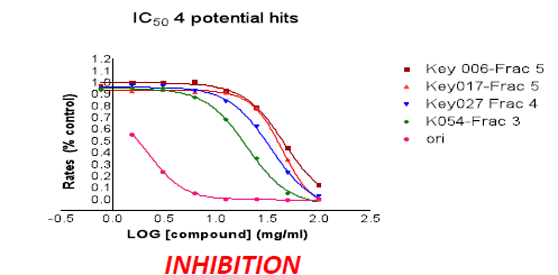
2) Collaboration with Chemists around the world - In addition to using animal models, the laboratory collaborates with chemists at McGill University and around the world in the development of ways to inhibit or regulate protein tyrosine phosphatases. We are screening small molecules in vitro and in cells for their specificity and efficacy to inhibit over 35 different members of the PTP family. We have also generated, for our five enzymes of interest, cell-based models and other reagents (antibody, siRNA, etc) that allow us to characterize their function and mechanism of action.
3) The Role of PTPs in human cancer - We are examining their role directly in cancer patients particularly in breast, prostate, pancreatic cancer and in leukemia. We are also looking at their role in the cachexia/wasting syndrome that afflicts nearly 40% of cancer patients which represents a major cause of morbidity for individuals with cancer.