Our Research
Drug resistance in multicellular parasites
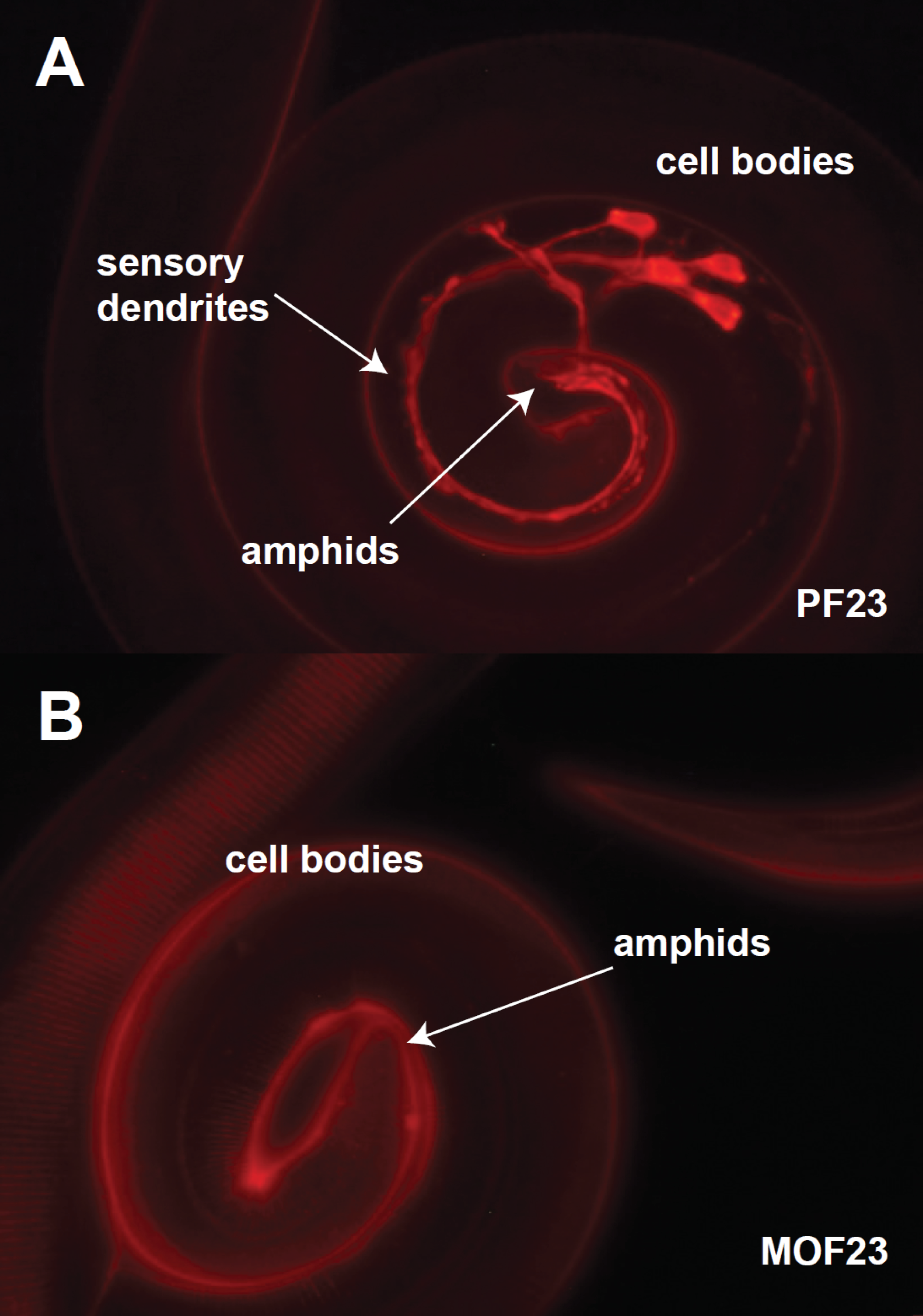
Roundworm (nematode) parasites represent a significant disease burden both in livestock and in human populations of developing countries. We rely on antiparasitic drugs, ivermectin being among the most important, to control roundworm infections in humans and to improve health and productivity in livestock. Notably, ivermectin is the cornerstone of mass drug administration programs designed to eradicate filariases in sub-Saharan Africa. Unfortunately, the ability of roundworms to evolve drug resistance has been demonstrated repeatedly and threatens compromise the efficacy of antiparasitic drugs faster than new drugs can be developed. A goal of our research program is to understand the genetic and mechanistic basis of ivermectin resistance, information that we hope will ultimately help to halt and reverse the spread of resistance in parasites and preserve the efficacy of our current drugs. We have worked primarily with with the model nematode C. elegans to understand the genetics of drug resistance and have translated those results to parasite species.Dye filling in an ivermectin sensitive (A) and an ivermectin resistant (B) of H. contortus. The sensory neurons of the drug-sensitive strain absorb fluorescent dye from the environment.The figure to the right shows two strains of the sheep gastrointestinal parasite Haemonchus contortus: the top panel is a drug-sensitive strain and the bottom panel is a drug-resistant strain. Both strains have been soaked in a red fluorescent dye that is absorbed into the sensory neurons in the sensitive, but no the resistant strain. We hypothesize that the sensory neurons are an important site of drug entry into the body of the worm and hence the association with drug resistance. A number of genes the affect dye-filling have been identified in C. elegans that are conserved in H. contortus.
Evolution of pentameric ligand-gated ion channels
Expression and subcellular localization of secCl chloride channel in the stellate cells of Drosophila Malpighian tubules. g) green staining is secCl antibody, blue is DAPI. h) red is CD8_mCherry, a marker of apical membranes. i) the merge of g and h.One of the surprising results of work on drug resistance is that many of the successful antiparasitic drugs have invertebrate-specific neurotransmitter receptor as their targets. Specifically, they target members of the pentameric ligand-gated ion channels (pLGICs) protein family that includes many of the major vertebrate neurotransmitter receptors including GABA, glycine and nicotinic acetylcholine receptors. We now know that invertebrates encode a much wider spectrum of pLGICs than vertebrates, including neurotransmitter receptors that have no equivalent (ortholog) in vertebrates. The evolutionary origin of these unusual invertebrate ion channels appears to be deep, going back to the origin of multicellularity and the evolution of the first nervous systems. To better understand how nervous systems evolved, we have characterized a number of these channels, including acetylcholine-gated chloride channels in C. elegans and channels in Drosophila that are not neurotransmitter receptors at all, but rather regulate secretion. The results suggest that neurotransmitter receptors evolved from channels that previously served to regulate ion secretion.