A para- to meta-isomerization of phenols
Phenols are a common functional group of many naturally occurring and biologically active molecules, including many that are part of the human diet. In 2020 alone, ~60% of small molecule drugs approved by the FDA contained a phenol or a closely related phenolic ether, underscoring their importance to the pharmaceutical industry.
The substitution pattern of a phenol, namely, the relative positioning of groups around its associated aromatic ring, has a critical role in determining how the phenol can engage with its surroundings. The substitution pattern effects both the bond dissociation energy and acidity of the O−H group, as well as the redox properties of the aromatic ring. As a result, phenol isomers can exhibit very different behaviours. For example, the para-isomer of L-tyrosine is a common amino acid, needed for protein synthesis and normal cell-function, whereas the meta-isomer is a toxin, used by some organisms as a chemical defense.
Given these differences, it could be interesting to explore the effects of a phenol isomerization. However, such a tool has not existed in the organic toolbox, requiring the de novo synthesis of any non-naturally occurring isomer. Likewise, the isomers of phenolic-drugs could be equally interesting to investigate from a medicinal chemistry perspective, but the need to synthesize the new isomer could be prohibitively time consuming or expensive.
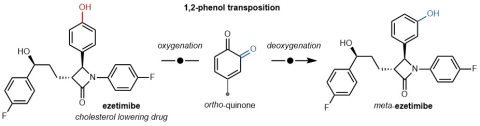
With this background in mind, the Lumb Group is now reporting a single-step methodology that moves the O−H group of a phenol to its neighbouring carbon, enabling for the first time, a para- to meta-isomerization. The process involves oxygenation of the phenol to an ortho-quinone, followed by selective deoxygenation to provide the transposed product. Amongst the many examples included in their recent publication, the Lumb Group highlights the isomerization of the cholesterol lowering drug ezetimibe (pictured in the attached figure) as a means of showcasing the method’s utility on complex, polyfunctional substrates, relevant to medicinal chemists. Beyond exploring the biological activity of new chemical space, this tool could also be useful to practicing organic chemists, interested in synthesizing phenols as part of longer synthetic sequences.
To find out more, visit the Lumb Group’s Website (https://www.lumblab.org/), and check out their recently published work in Nature Chemistry:
“A para- to meta-isomerization of phenols”, by Simon Edelmann and Jean-Philip Lumb was published in the April 2024 issue of the Nature Chemistry. (https://rdcu.be/dE0vi)