Peter Siegel, Ph.D.
Professor/Professeur
Rosalind and Morris Goodman Cancer Institute
Department of Medicine
Faculty of Medicine and Health Sciences
McGill University

1. Research Scholar of the Canadian Cancer Society, 2004-2010
2. Research Scholar of the FRQS – Junior 2, 2010-2012
3. William Dawson Scholar, McGill University, 2014
RESEARCH OVERVIEW: The Metastatic Cascade
Metastatic tumor cells must overcome numerous barriers that prevent their dissemination to distant organs and tissues. Traditionally, these steps have been broken down to include local invasion through the basement membrane, migration and intravasation into the lymphatic or hematogenous systems, survival during transit in the circulation, evasion of host anti-tumor immunity, adhesion/extravasation at the target site and reestablishment of a growing tumor mass in a foreign microenvironment. While any metastatic tumor cell must fulfill these basic requirements, it has long been appreciated that certain types of cancer preferentially metastasize to specific organs and tissues. Indeed, breast cancer cells are known to frequently spread to regional lymph nodes, bone, lung, liver and brain. Several factors are likely to contribute to organ preference during breast cancer metastasis, including the pattern of blood flow leaving the primary tumor, physical entrapment of tumors cells in capillary beds within the metastatic site, tumor/endothelial cell interactions and the ability of tumor cells to productively influence and respond to the metastatic microenvironment. Communication between the tumor and its microenvironment is likely to be the single most important determinant for organ-specific metastasis. Thus, both tumor intrinsic and microenvironmental factors play important roles in controlling breast cancer metastasis.
RESEARCH FOCUS
We have established a comprehensive research program to the study the molecular mechanisms that control the metastatic process in breast cancer. Current research ongoing in my laboratory encompasses three broad research areas, which are briefly outlined below.
I. Interplay between TGFβ and ErbB2 signaling pathways in breast tumor progression and metastasis
We and others have shown that TGFβ signaling can promote the invasive and metastatic abilities of breast cancer cells using cell-based and transgenic models of ErbB2-driven breast cancer. Indeed, TGFβ modulates the actin cytoskeleton and focal adhesion dynamics in cancer cells, leading in enhanced migration, invasion and metastasis, through divergent mechanisms. Ongoing projects are focused on signaling proteins that lie downstream of these pathways to promote the pro-invasive and pro-metastatic effects that TGFβ induces in ErbB2-expressing breast cancer cells.
II. Mediators of organ-specific breast cancer metastasis
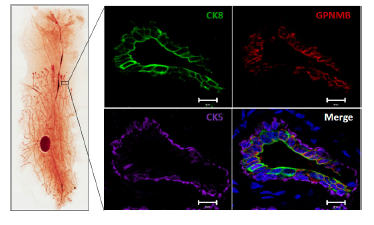
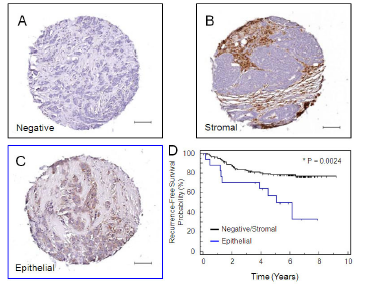
Another approach we have taken is the identification of molecular mediators of breast cancer metastasis to distinct sites. Organ-specific breast cancer metastasis has been investigated through the injection of human breast cancer cell lines into immunocompromised mice. This approach allows distinct sub-populations of metastatic cells to be isolated from particular organs or tissues. Gene expression profiling has revealed distinct genes that are characteristic of breast cancer cells that metastasize to several different organs. Subsets of genes within these organ-specific signatures have been functionally validated in xenograft models or compared to gene expression data sets derived from human breast tumors. We have extended this approach to the 4T1 mouse mammary carcinoma cell line since it spontaneously metastasizes to distinct organs and tissues, including the bone, lung, lymph node, liver and brain following orthotopic injection into the mammary fat pads of Balb/c mice. We have used this cell model to identify genes that are associated both with a general metastatic ability (GPNMB) and those associated with breast cancer metastasis to bone (CCN3) and liver (claudin-2).
III. The influence of the metastatic microenvironment on breast cancer metastasis
The use of in vivo selected breast cancer populations has been very useful in identifying tumor intrinsic gene expression profiles from which functional mediators of the metastatic process can be identified. However, this approach does not capture gene-expression changes induced in breast cancer cells in response to the metastatic microenvironment. To address this, we have taken the approach of performing laser capture microdissection on metastases in the liver and bone from patients with breast cancer. The identification of candidate genes directly from metastatic tissue increases the likelihood that identified targets are relevant to the human disease and we can functionally validate their role in the metastatic process using the in vivo selected cell-based models described above.